OPTON Micro World | Application of the 26th SEM in Na Ion Batteries
1 Na battery background
Lithium-ion batteries have become the first choice for energy storage in the mobile electronics industry, from mobile phones to laptops to cars. However, its electrode material contains lithium, which is rare in the earth. The cathode material cost of lithium-ion batteries accounts for 30% of the battery production cost. Over the years, people have been looking for alternatives that are cheaper and cheaper. Na-ion batteries are suitable for large-scale grid energy storage systems due to their significant cost advantages and environmental advantages. In order to meet the requirements of large-scale energy storage devices, it is necessary to develop a sodium ion battery material having both high energy density and recyclability. The layered metal oxides currently used in cathode materials for sodium ion batteries have high energy density, but have poor cycle stability and are sensitive to air. Compared with organic compounds, cathode materials can be simple from biomass materials. The processing is obtained with a price advantage, but how to improve its energy density and long cycle stability is still a great challenge.
2 Na battery difficulties
Among the many organic electrode materials, sodium palmitate (Na2C6O6) is a promising cathode material for sodium ion batteries with high theoretical specific capacity (501 mAh/g) and long cycle stability, and can be extracted from plants. The inositol starts and is prepared at a lower cost. However, the reversible capacity of sodium alginate as a sodium ion cathode material is much lower than its theoretical capacity, and it is accompanied by significant capacity decay during the first cycle. The theoretically expected four-electron process is difficult to achieve in practice, resulting in the above. The deep-seated reasons for the phenomenon are unknown.
3 Na battery mechanism research
Recently, Professor Bao Zhelan and Professor Cui Wei from Stanford University used SEM and XRD instruments to change the structure of charging and discharging processes of different sizes of sodium sulphate materials under different electrolytes.
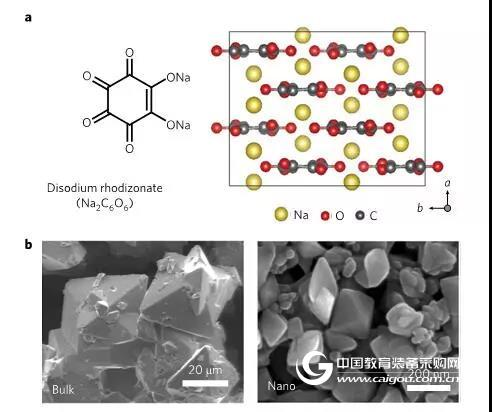
Figure 1 (a) Chemical formula of sodium palmitate (Na2C6O6) and SEM characterization of the corresponding atomic structure (b) and nanoscale sodium palmitate
The change of the in-situ XRD diffraction peak position shows that the structure of sodium palmitate (Na2C6O6) changes between γ-Na2.5C6O6 and α-Na2C6O6 during charge and discharge. With the Na process, from the transformation of the original γ-Na2.5C6O6 to α-Na2C6O6, a large amount of activation energy needs to be overcome, and the phase transition exhibits large irreversibility, thus seriously restricting the electrochemical properties of the material. performance. A schematic diagram of the phase transition occurring during the decanting process is shown in FIG.
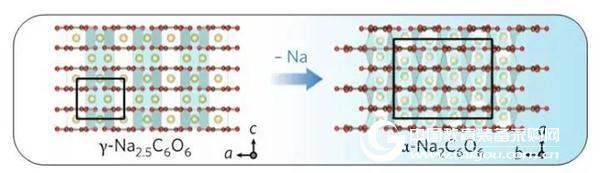
Figure 2 Sodium alginate (Na2C6O6) is converted from the original γ-Na2.5C6O6 to α-Na2C6O6 with the Na removal process.
In further experiments, the application of the nanometer-sized sodium palmitate with DEGDME (diglyme) electrolyte significantly reduced the activation energy barrier of the phase transition, so that γ-Na2.5C6O6 during discharge The phase transition with α-Na2C6O6 is highly reversible, and the Na-reservation mechanism of reversible storage of 4 Na atoms in each Na2C6O6 unit cell is realized, thereby achieving high reverse capacity and cycle stability. Figure 3 shows an SEM image of the morphological changes of nano-sized sodium palmitate particles during charge and discharge of the DEGDME electrolyte.
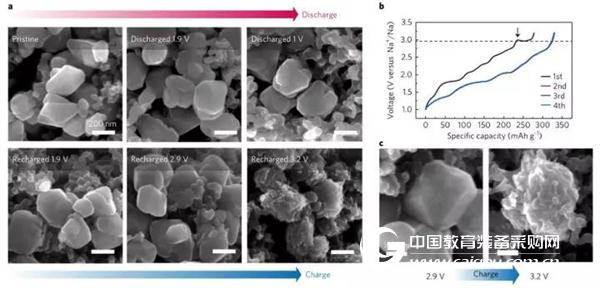
Figure 3 (a) Rear view of SEM morphological observation of nanoscale sodium palmitate (Na2C6O6) in various stages of charging in DEGDME electrolyte (b) Nanoscale sodium palmitate was charged four times in DEGDME electrolyte Charging curve (c) The process of dynamic morphology of nano-sized sodium palmitate particles during charging (2.9V and 3.2V)
Finally, a schematic diagram of the positive electrode cycle of sodium palmitate (Na2C6O6) is given. As shown in Fig. 4, it can be seen that the mechanism of storing Na atoms directly affects the energy storage efficiency.
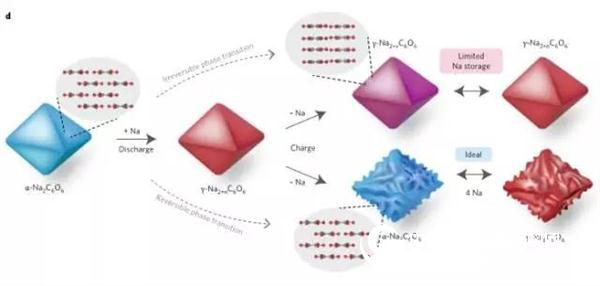
Figure 4 Schematic diagram of sodium and sodium removal mechanism of sodium succinate (Na2C6O6) cathode
Summary of 4 Na battery mechanism
For the first time, the researchers revealed the root cause and influencing factors limiting the electrochemical performance of sodium palmitate (Na2C6O6) as an organic cathode material for sodium ion batteries. Based on the in-depth study of the mechanism of electrode reaction and the evolution of the morphology and structure of the electrode material, it is explained that the reason for the poor cycle performance and low specific capacity of sodium alginate (Na2C6O6) is that the phase transition is irreversible during charge and discharge. Sex. By reducing the grain size and the appropriate electrolyte in the selected area, the activation energy of the phase change is reduced, thereby promoting the phase change process during the charging process, thereby improving the reversibility of the phase change, and improving the sodium alginate (Na2C6O6) cathode material. Electrochemical performance.
postscript
The above research on the change of the morphology of the positive electrode material sodium succinate (Na2C6O6) in the charging process is based on the observation of SEM electron microscopy. It can be said that the SEM realizes the characterization of the material detail changes, confirming that the material is in the phase transition process. The morphological changes that occur, combined with the corresponding structural changes in the material, strongly explain the mechanism of the Na-ion battery. Therefore, it can be explained that SEM technology has wide application value in nano materials and new energy materials.
Related papers link: High-performance sodium–organic battery by realizing four-sodium storage in disodium rhodizonate (Nat.Energy, 2017, DOI:10.1038/s41560-017-0014-y)
6 Options for home office storage
Whether it is a place for getting work done or simply open your mail, your home office furniture should deliver comfort and productivity.
Metal furniture manufacturers have been working hard to create furniture combining both form and function.
1. Metal Wardrobe cabinets are a great way to keep personal belongings or uniforms.
2. Metal Chest Of Drawers run smoothly on metal glides, offering easy access to storage for clothing and bedroom items.
3. Half height Metal Cupboards are used as Home Office Cabinets for personal belonging storage.
4. A durable Locker TV Stand cabinet can fit almost anywhere , living room or a home office.
5. Metal Locker Night Stand adds a fun vibe to your space.
6. Open metal home racks not only allow you to find things easily, but also because they are on display for everyone to see.
Metal beds and cabinets can be combined to create a highly durable, highly functional living space.
Metal Dressers ,Metal Wardrobe Price,Metal Locker Tv Stand
LUOYANG SHIDIU IMPORT AND EXPORT CO., LTD , https://www.shadowcabinete.com